blog
RPO Group flyer
Download our flyer to get to know RPO Group in a nutshell.
Read more

blog
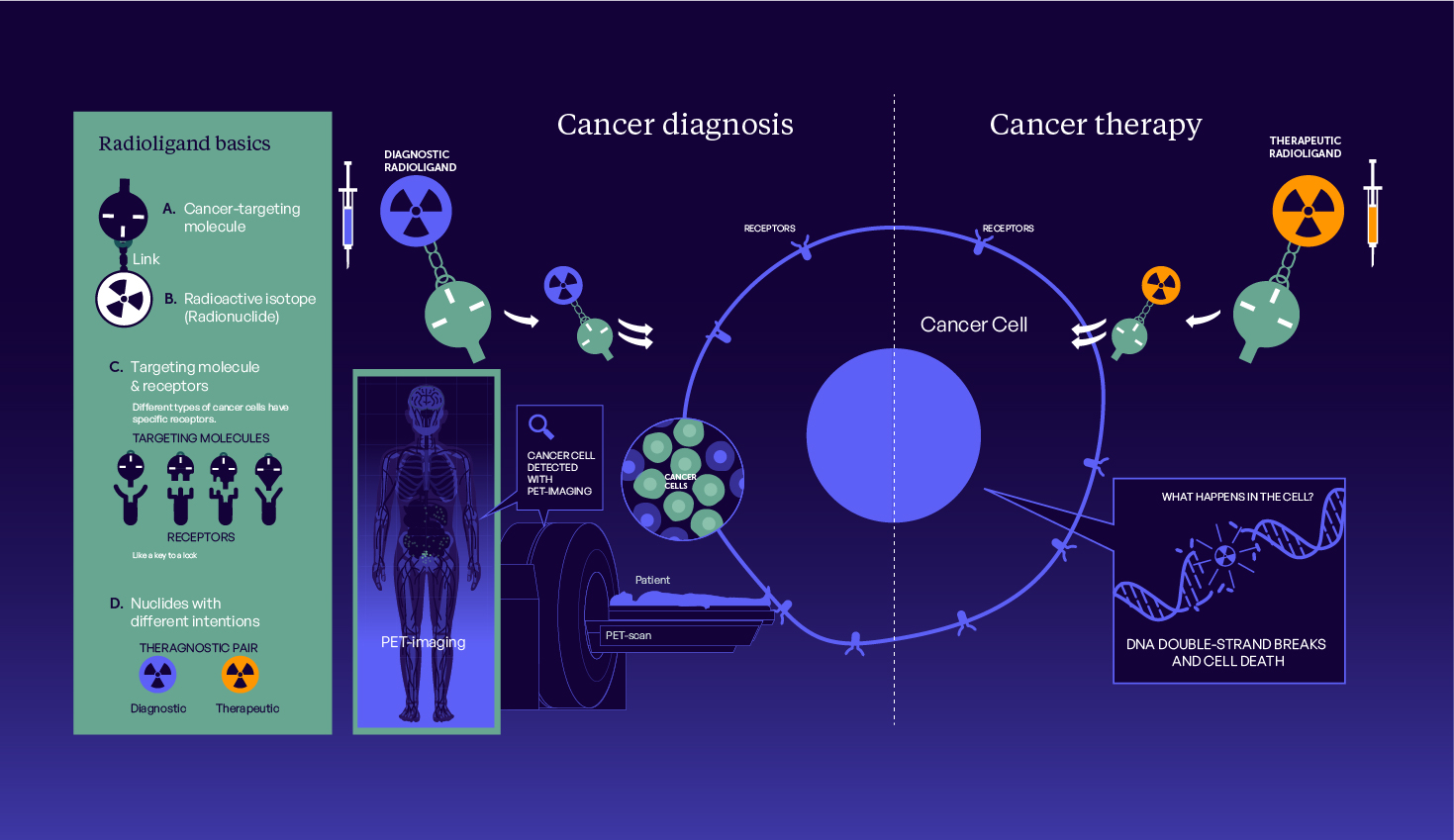
Theranostics explained
Download our infographic to see how Theranostics can tailor treatment to your unique health profile, enhancing effectiveness and minimizing side effects.
Read more

blog
RPO Group will be attending BNMS Annual Spring Meeting
On 13-15 May RPO Group will be attending BNMS Annual Spring Meeting in Belfast.
Read more